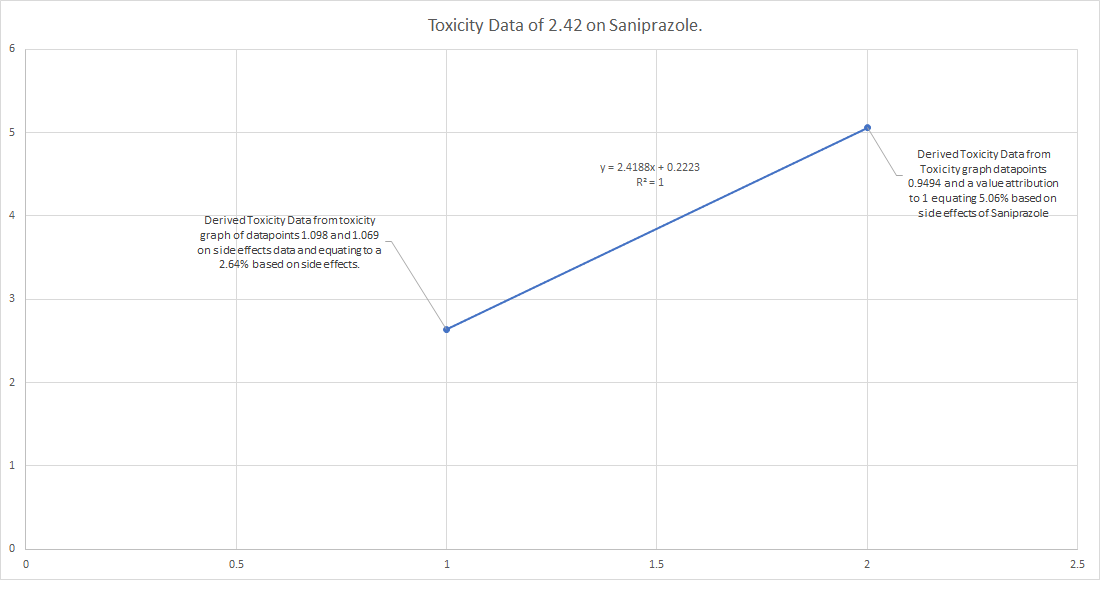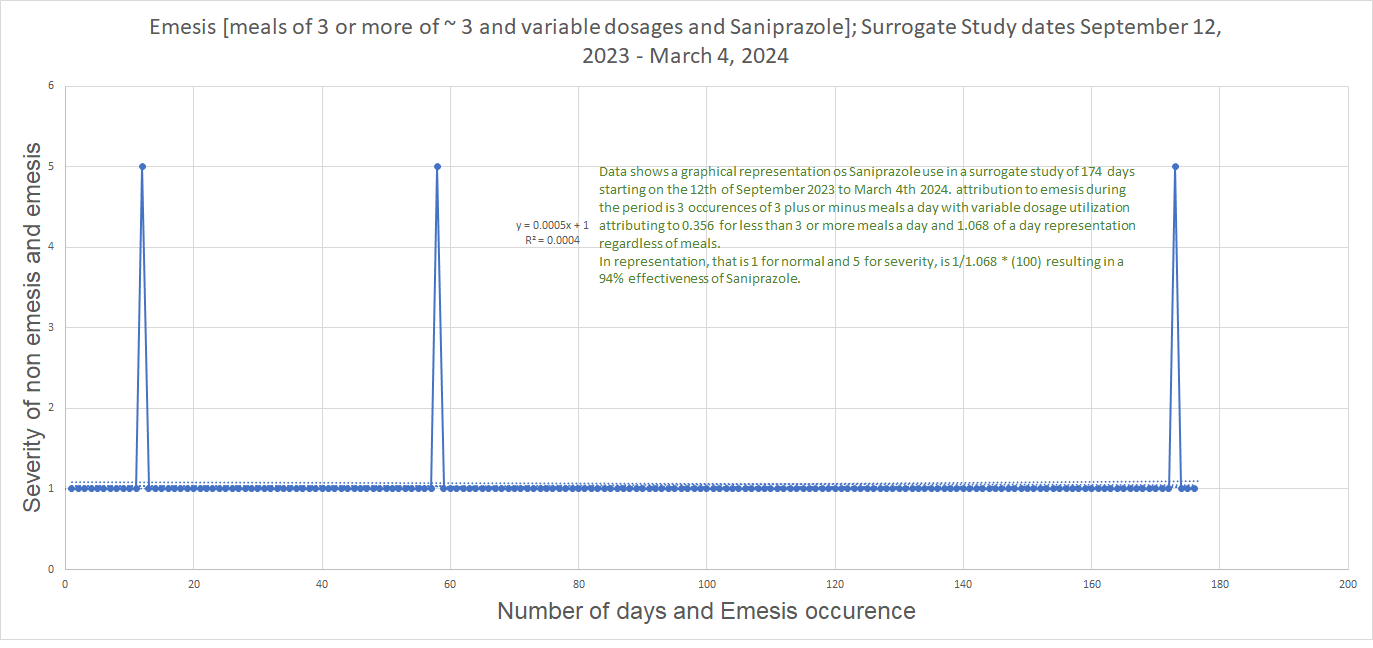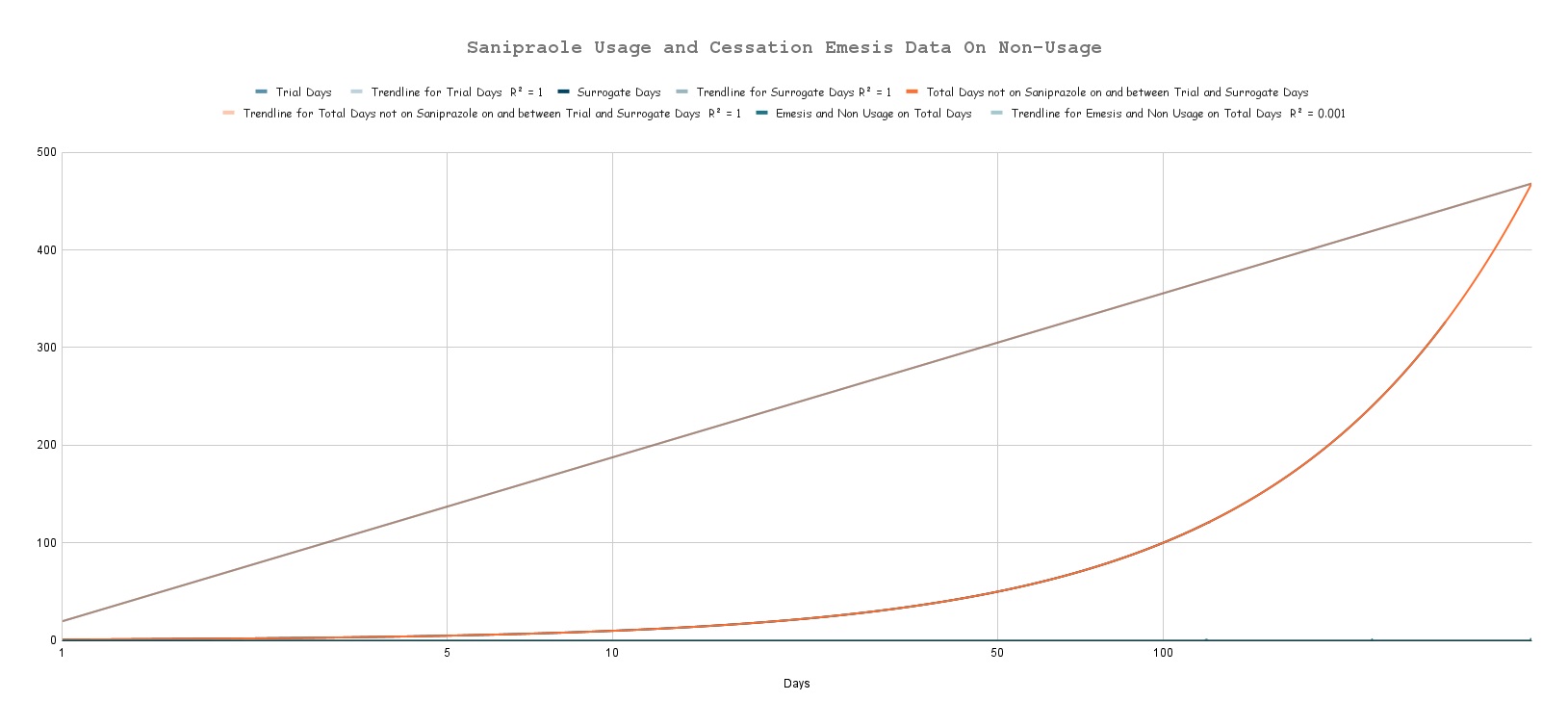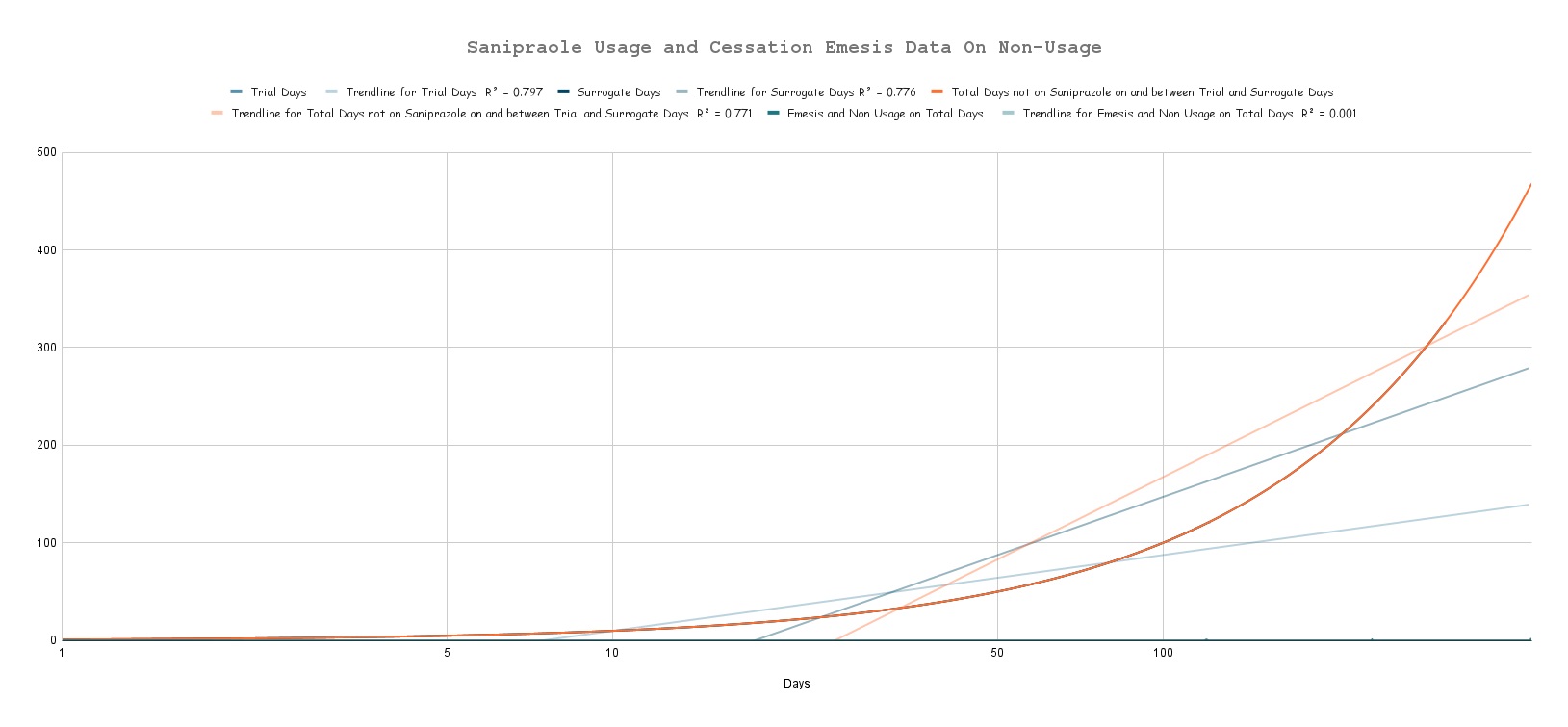

Illustration of the Digestive System by Da Vinci; DIO - A tunnel for life!
SANIPRAZOLE 
hUH? IT WORKS, THIS WORKS?! Yuh!
Saniprazole mkII and Saniprazole confirmed on monitoring for reemission for EoE on study completion already turned in to FDA; Emesis confirming 3 months Reemission study profile October 28th 2024- January 5th 2025.
In confirmation of a 3 months remission to study and trial collated, in review of data and non- collation of data but monitoring and observing, remission study confirmed for saniprazole and mkII emesis stop period of October 28th 2024 to January 5th 2025 and January 27th 2025; confirms a 4 month remission to cessation of Saniprazole and a month on subsequent emesis remission for a sum total of 5 months for emesis, a major symptom remission.