DIOIC BIOLOGICS
Realizing a Future.
DIOIC Pipeline
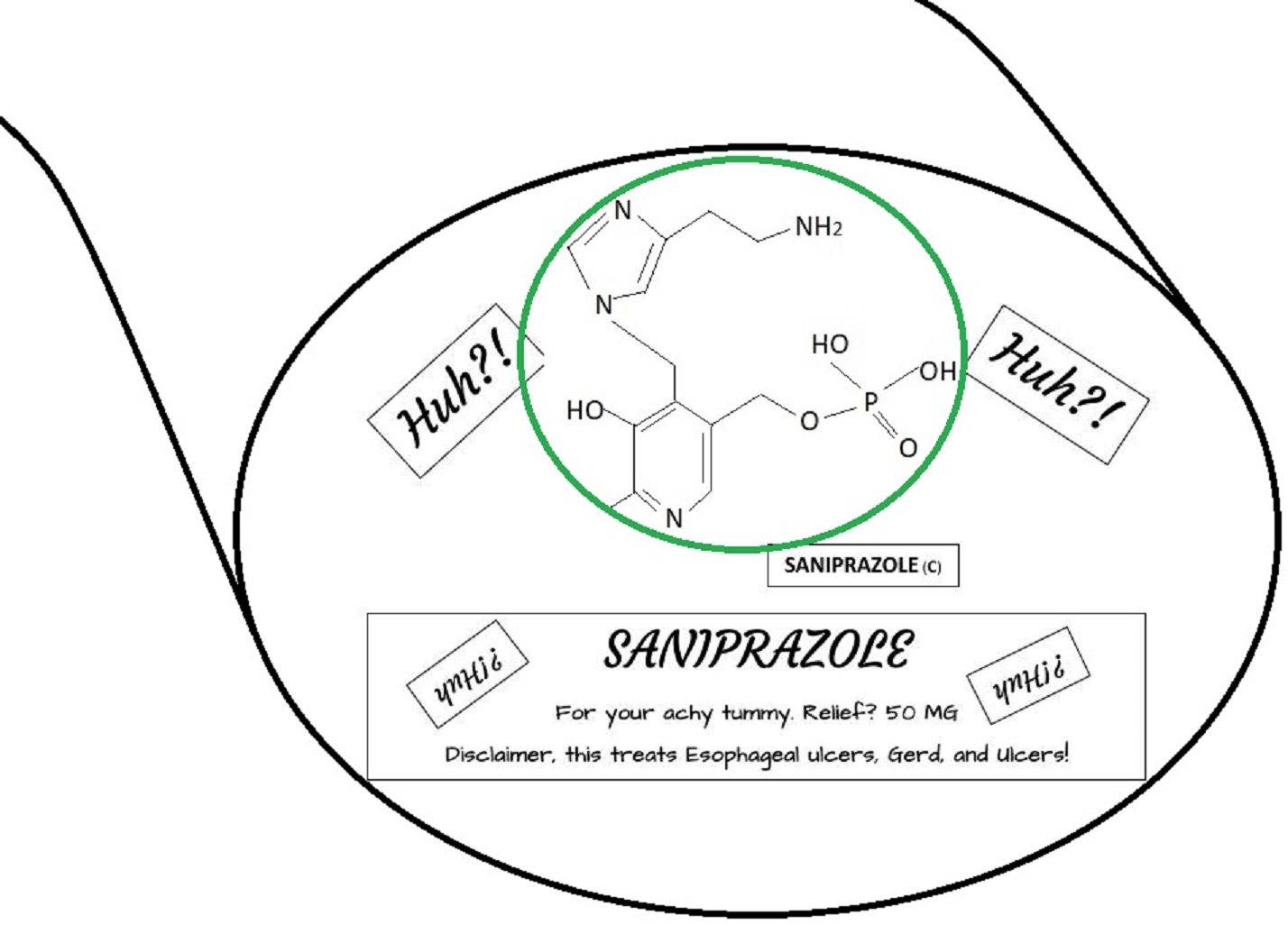
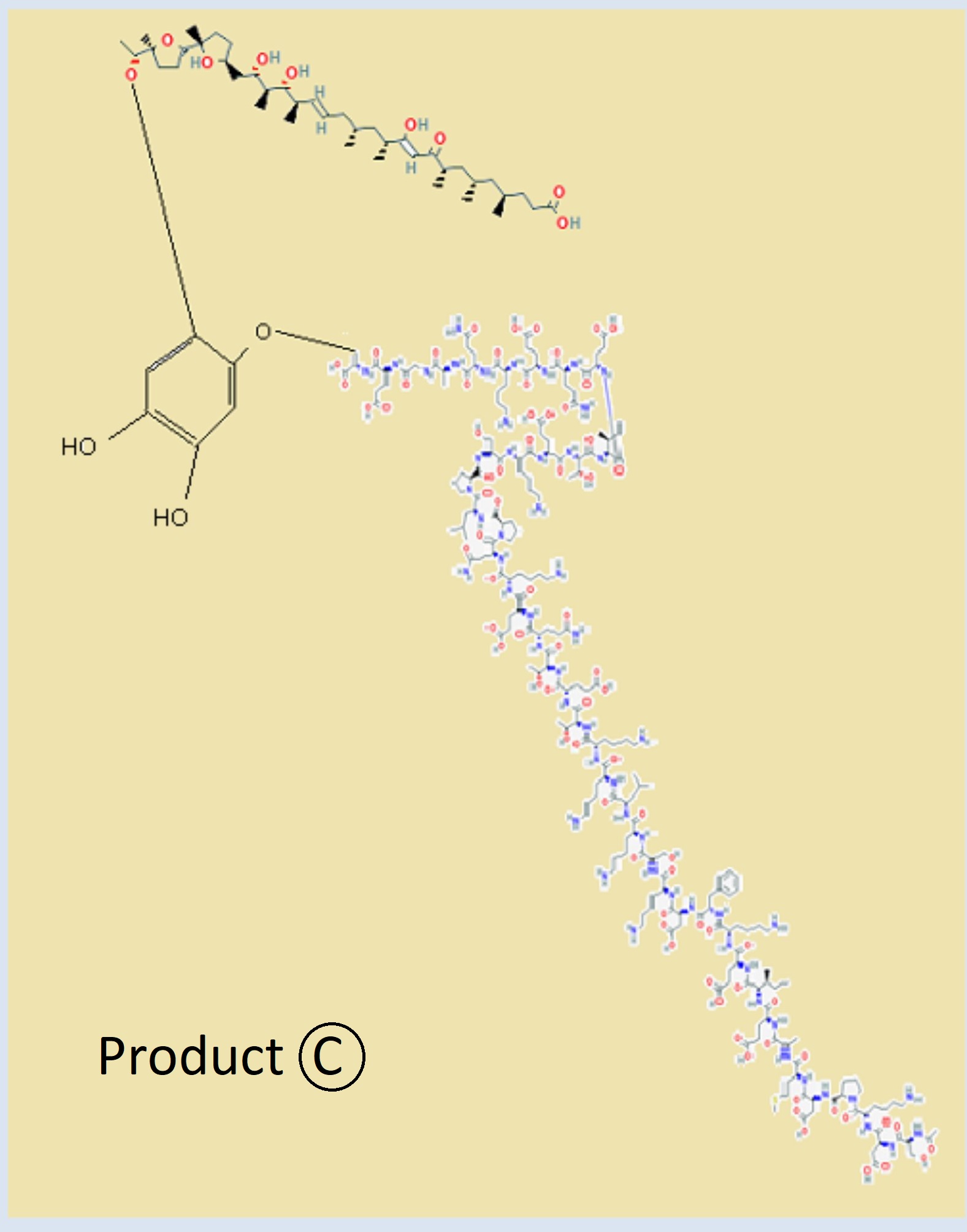
Saniprazole Mk2.
Preminilary Test phase; Passed and Under FDA Review for Approval on fast track
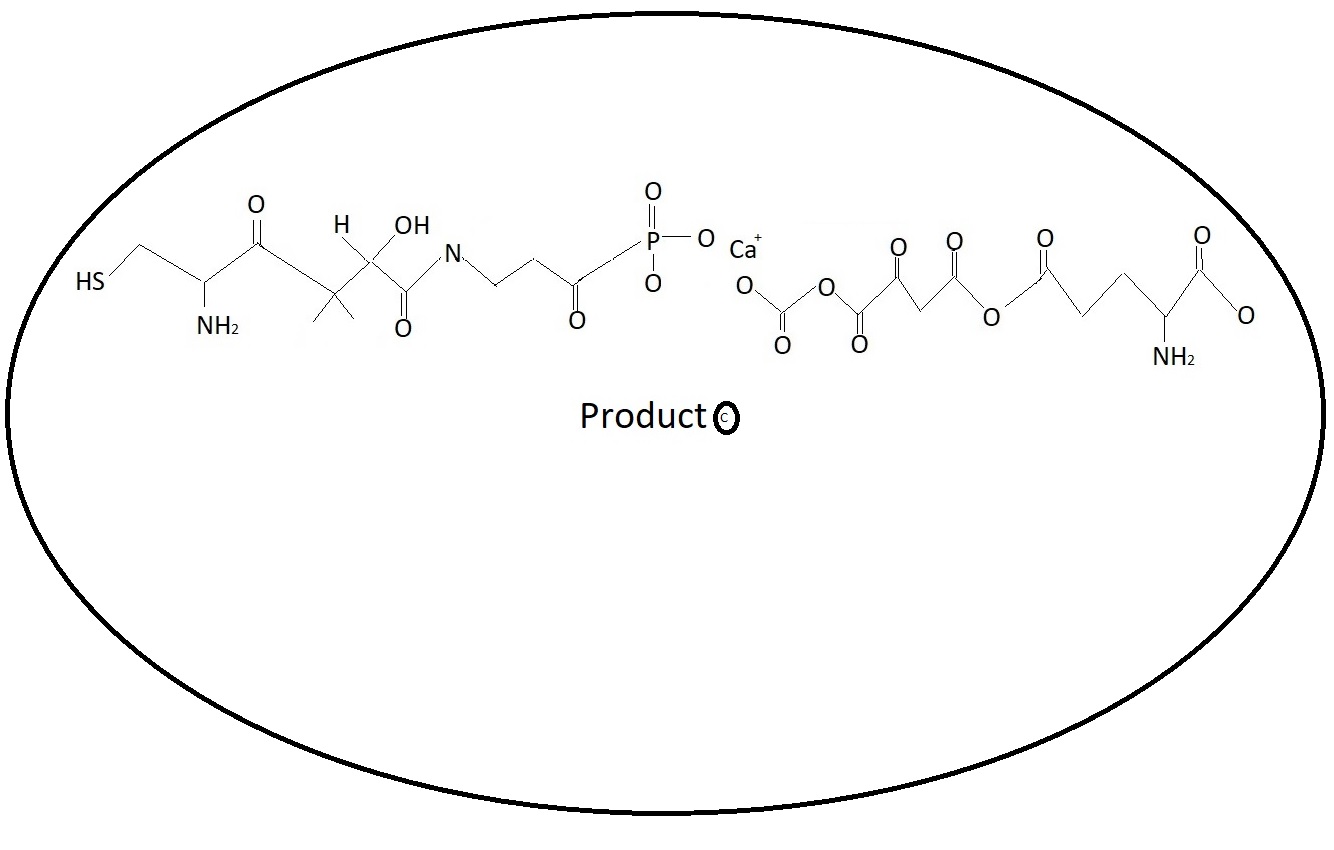
Muscle Dystrophy - Discovery Phase.
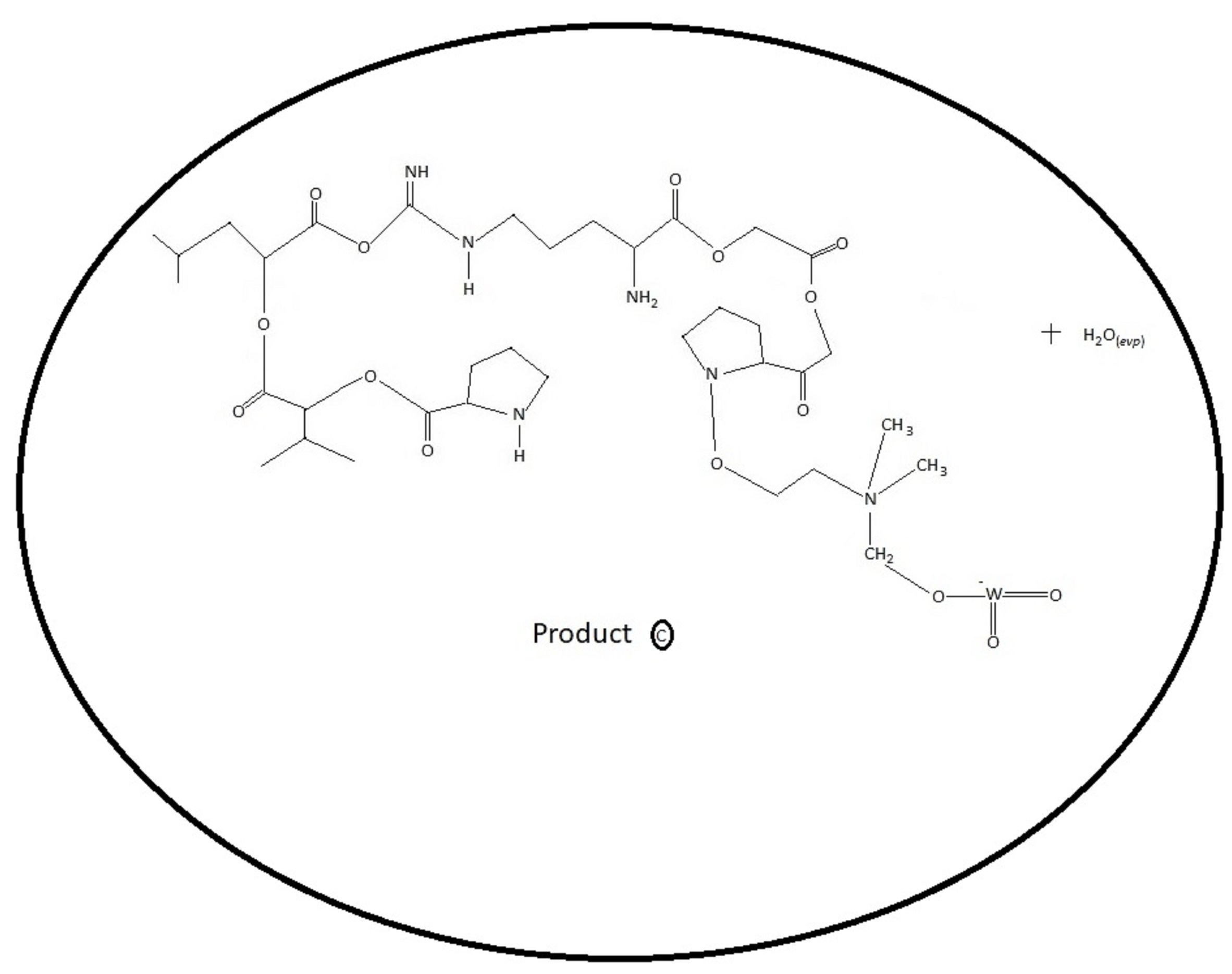
Thrombocytopenia - Discovery Phase.
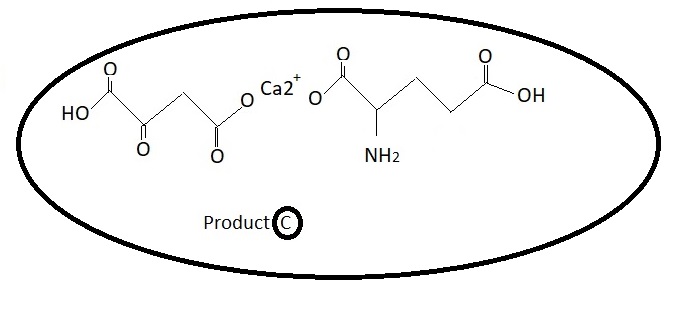
Diabeties 1 - Discovery Phase.
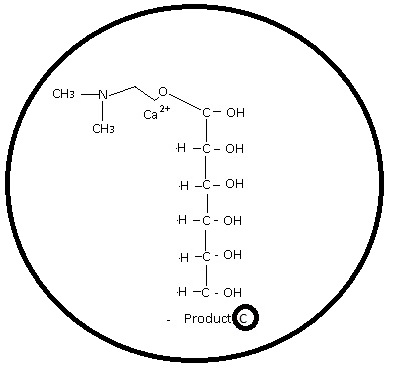
Owa1Cardiomegaly - Discovery Phase.
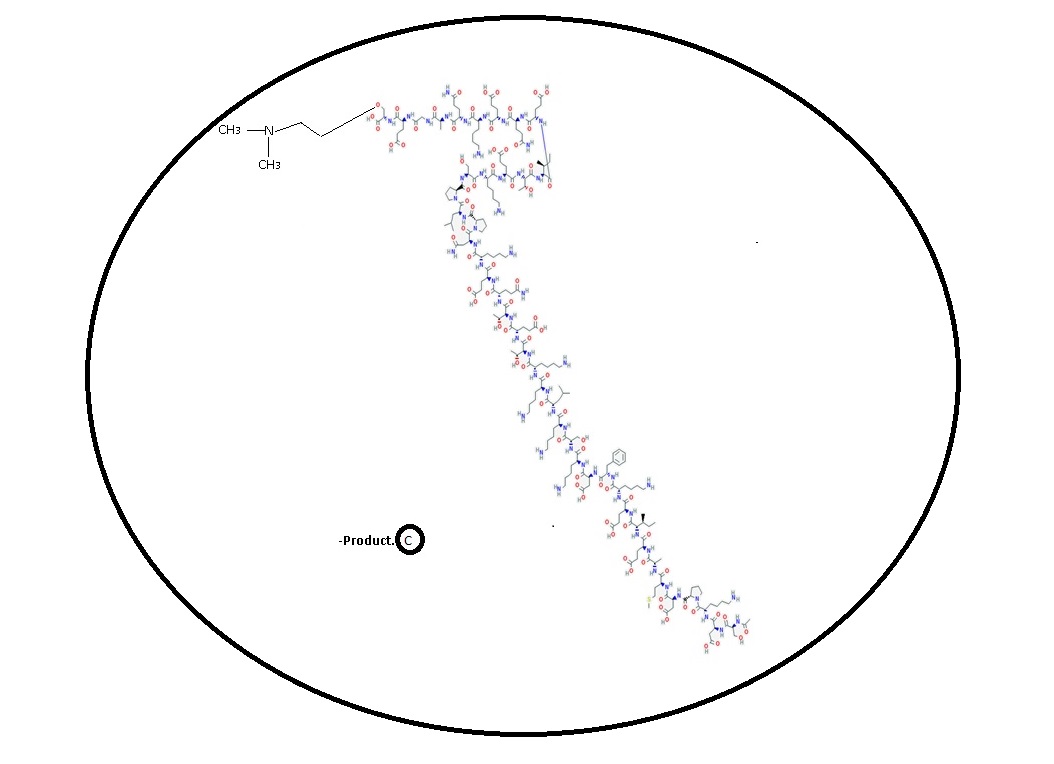
Owa2Cardiomegaly Diabetes2 - Discovery phase.
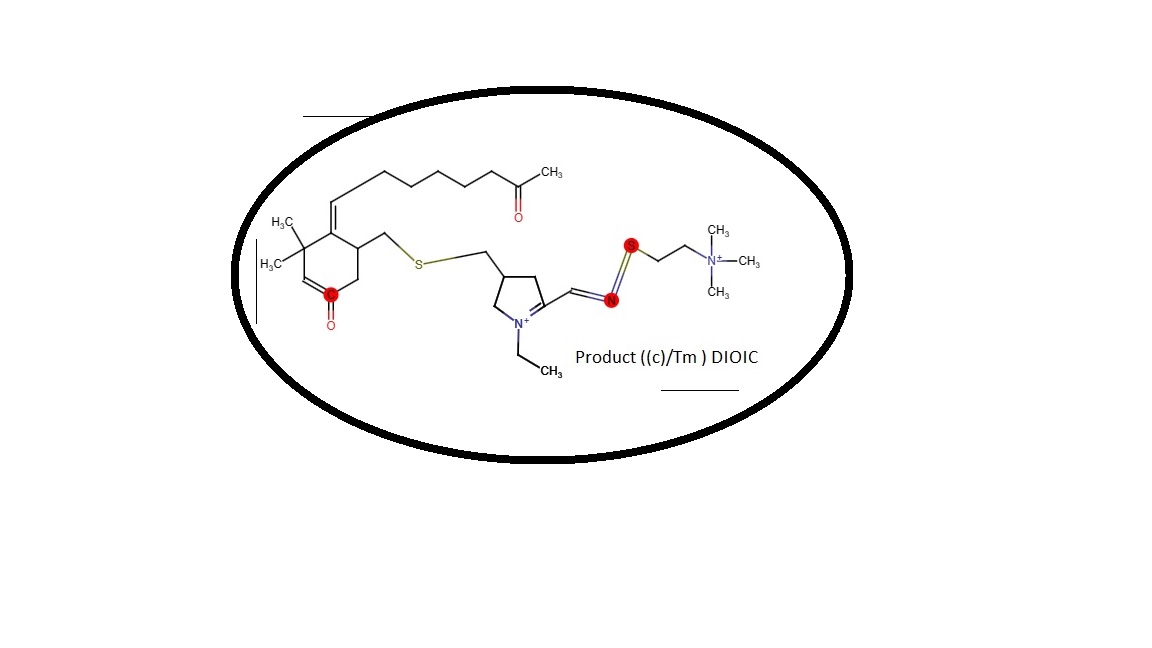
Schizophrenia / Bipolar Drug - Discovery Phase.
DIOIC Biologics Drug Publication of Trials and Studies.
Gastreointestinal Publication on EoE and Gerd with Saniprazole and Saniprazole mkII
DIOIC Biologics News
Current Developments with DIOIC Biologics with FDA CDER Next Gen and ESG in submissions for INDs and NDAs made on July 16 2025; and a pre-assignment for 4th generation psychotic medication submitted on 4th of August 2025 and approved for on the 5th of August 2025.
Current Development in conjunction with DIOIC ThinkTank towards regulatory approvals and streamlining the FDA process.
Current Developments with DIOIC Biologics; applications for Ind and NDA grant request to the FDA CDER Next Gen INDs and NDAs. Responded to Abeyance letters for Saniprazole, Saniprazole mkII and Emepi 6/24/2025.
Current Developments with DIOIC Biologics; applications for Ind and NDA grant request to the FDA CDER Next Gen INDs and NDAs. 6/23/2025.
Current Developments with DIOIC Biologics; applications for Ind pre Assignment granted by the FDA CDER Next Gen INDs and NDAs. 6/23/2025.
Data showing the treatment of Saniprazole and Saniprazole mkII to Gastreointestinal Ailments Eoe Gerd and Ulcers.
Current Developments with DIOIC Biologics with FDA CDER Next Gen in Acknolodgement of INDs and NDAs Ophan status.
Current Developments with DIOIC Biologics with FDA CDER Next Gen Addendums and INDs and NDAs
Current Developments with DIOIC Biologics with FDA CDER Next Gen
Current Developments with DIOIC Biologics with WHO
Current Developments with DIOIC Biologics
Correspondence with the Food and Drug Administration regarding delays in application approval.
Current Developmets: Updates to drugs regulatory body BfArm
Current Developments
DIOIC Biologics Business Developments - Acquisition
DIO Industries Corporporation is currently in negotiation in provision of an offer for Acquisition of FogHorn Therapeutics Inc [FHTX] and have made contact in regards towards a full acquisition of FogHorn Therapeutics INC . 20th of December 2024.
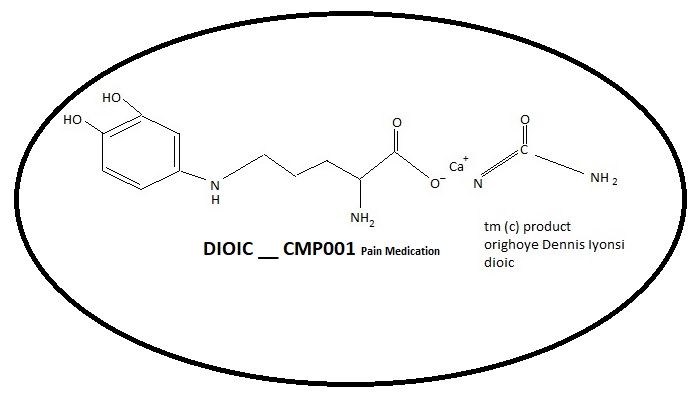
Purchase Requests with EMEPI - CMP001 Current Developments
DIOIC Biologics Regulations and Business News Emepi - CMP001.
Filled and Submitted an IND and NDA with the FDA with the DIOIC's Contact with the FDA.
DIOIC Biologics Regulations and Business News.
DIO Industries Corp Biologics submitted applications for drug candidates Saniprazole and Saniprazole mkII for a fast track approval on the 16th of August 2024, with all pertinent information provided to. In submittal for IND and NDA approval submissions was a concurrent study on Anagelsic effects on a patient on a Gastrointestinal medication grant request which was also turned in on the 24th of September 2024. With Study/Trial conclusions to Saniprazole, Saniprazole mkII and Analgesic study of a Gastreointestinal medication also submitted officially on the 31st of October 2024. In business related association to DIOIC, an official bid was turned in for Kimia Farma, Indonesia and BioAmerica for Acquisitions.
DIOIC Biologics Business Developments - Acquisition
DIO Industries Corporporation is currently in discussion with the Indonesian Government in a partial Acquisition of Kimia Farma and have made contact in regards, towards a partial controlling acquisition of Kimia Farma. 17th of September 2024.
DIOIC Biologics Business Developments - Acquisition
DIO Industries Corporporation is currently in a preminilary Interest in Acquisition of BioAmerica Incorporated and have made contact in regards, towards a full acquisition of BioAmerica Incorporated. 12th of September 2024.
Successful Endpoint Remission Study!!
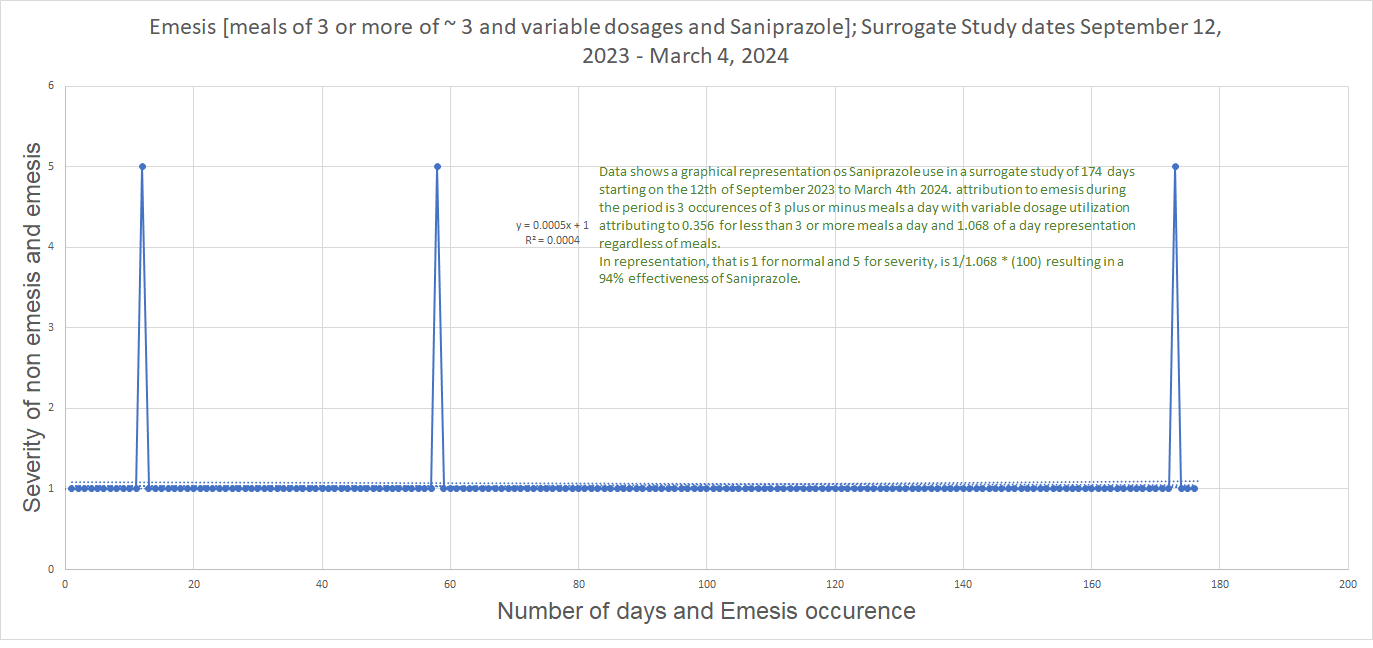
DIO Industries Corp is currently undergoing a 7 - Day Remission surrorogate study, i.e a continous evaluation of Saniprazole in cessation usage to determine remission probability subjected to a time frame. This study commmenced on the 25th of January 2024. Conclusive result to the 7-Day study ending on Day 143, Wednesday January 31st, 2024 of the Surrogate study exhibited a zero[0 - 1] scale rating for normalcy suggesting a zero percent [0%] remission investigation and a reaction of inducive coughs with a rating of three [3] of a scaled [(0-1) of 5] with two [2] occurrences in the 7-day concurrent trial, suggesting a [28.6%] reactionary exhibition off Saniprazole on the 7-day study. Primary endpoint interpretation shows zero remissions to symptoms associated to Gastrointestinal conditions like EoE and Gerd and secondary endpoint of reactions in coughs at [28.6%].
Surrogate Study Data on predominant condition in Eoe and Severe Gerd in relation to Emesis.