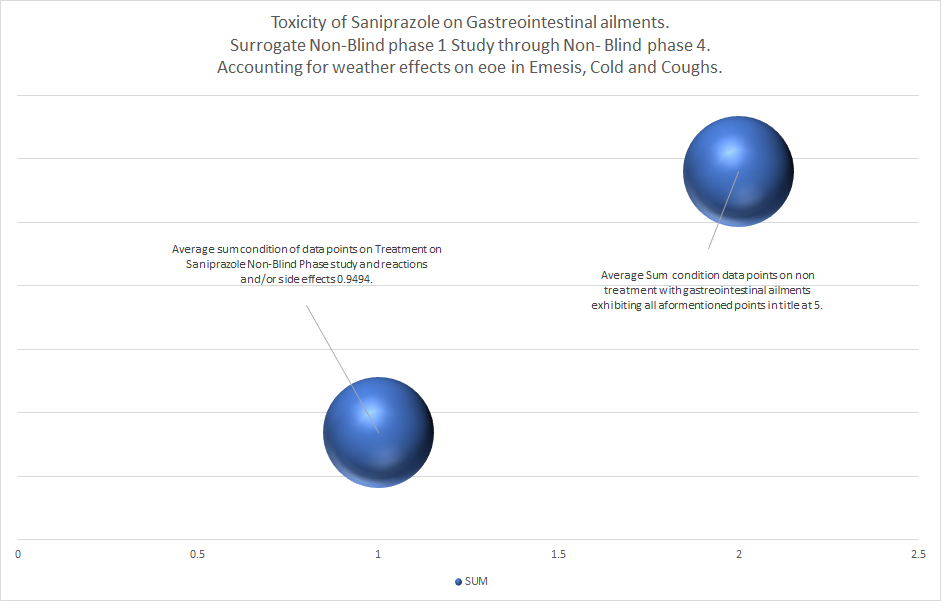
In utilization of formulation compound, Saniprazole, for conditions specified in daily processes with both food and drinks, exhibiting an increased association to conditions highlighted with regards to toxicity, utilization shows little to absolutely no exhibition of condition warranting a medical or health personnel with a value attribution of 2, in line with daily utilization of non-toxic compounds.

