
In representation of SANIPRAZOLE ascertaining dosage stability with use frequency from a stalled utilization for determined ailment in EOE, GERD and Ulcer of perculiarity, EOE.
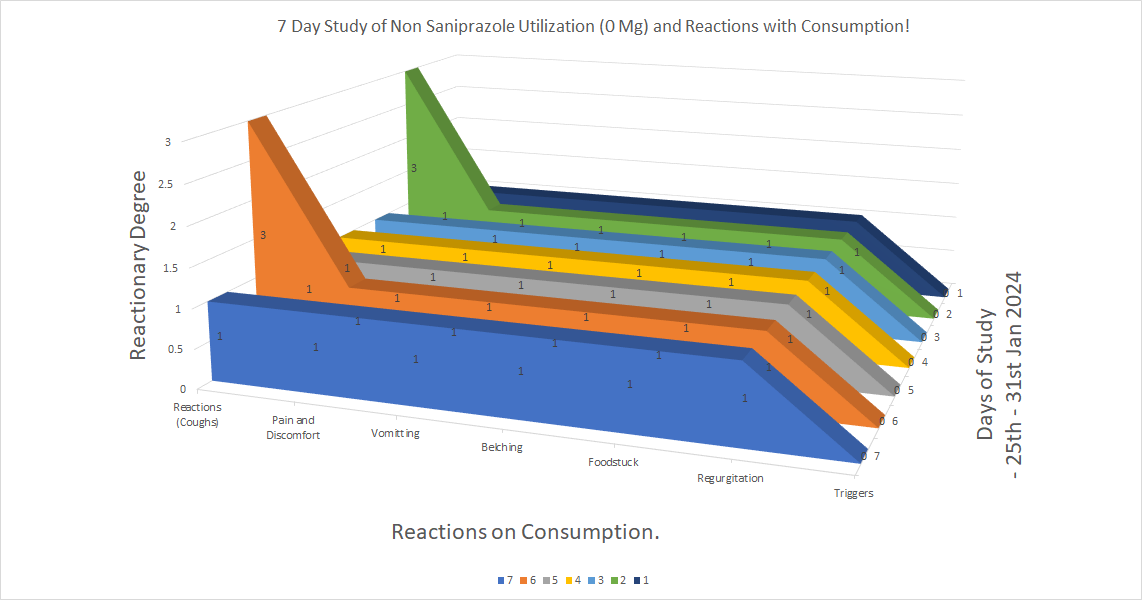
The Concurrent study carried out to the effectiveness and functionality of Saniprazole in cessation shows or coincides with periods of reactionary rise, denoting 3 for coughs on days when consumption involved allergic diets in Seafood in particuliarity to EOE, GERD and Ulcer.

